Disaccharides:
The ketone or aldehyde set on the anomeric carbon atom of one monosaccha- ride can react with the hydroxyl group of a second monosaccharide to form a disaccharide. The covalent bond established is known as a glycosidic bond. Lactose is a disaccharide formed among the anomeric carbon (C-1) of D- galactose and C-4 of D-glucose. Because the anomeric carbon of the galactose molecule is included in the bond and is in the configuration, this is known as a (1→4) bond that can be abbreviated as 1-4. Maltose is a disaccharide formed among the C-4 and C-1 positions of two glucose units. Moreover, here the configuration of the anomeric carbon atom included is the form and thus the bond is known as an (1→4) bond or abbreviated as 1-4. Intended for lactose and maltose, one of the anomeric carbons has been used to form the bond leaving the second anomeric carbon free. Therefore both lactose and maltose have a reducing end. In compare, sucrose is a disaccharide formed through bond formation among the anomeric C-1 of glucose and the anomeric C-2 of fructose so which sucrose lacks a free reducing group.
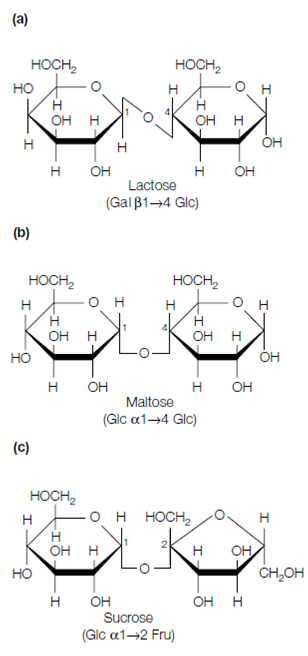
Figure: Structure of common disaccharides