Aldoses and ketoses:
A carbohydrate is composed of carbon (carbo-), and hydrogen and oxygen (-hydrate). The simplest carbohydrates are the monosaccharides that have the general formula (CH2O)n where n is 3 or more. A simple sugar or monosaccharide, consists of a carbon chain with a number of hydroxyl (OH) groups and either one aldehyde group frequently written as -CHO) or one ketone group. A sugar which bears an aldehyde group is known as an aldose whereas a sugar with a ketone group is a ketose. The smallest carbohydrates, for that n = 3, are known as trioses. The terms can be combined. Therefore glyceraldehyde is a triose which has an aldehyde group and so is an aldose. Therefore it can also be called an aldotriose. Similarly, dihydroxyacetone shown in the below figure is a ketotriose.
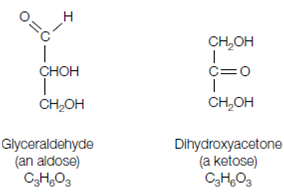
Figure: Structures of glyceraldehyde and dihydroxyacetone.
Sugars which contain a free ketone or aldehyde set in the open-chain configuration can decrease cupric ions (Cu2+ ) to cuprous ions (Cu+) and hence are called reducing sugars. This is the general of the Fehling's and Benedict's tests for reducing sugars. The decreasing end of such a sugar chain is therefore the end which bears the ketone or aldehyde group.
Note that glyceraldehyde and dihydroxyacetone have the same chemical composition, C3H6O3, but differ in structure (i.e. they are structural isomers).