Intermolecular forces
Among the charged ions (whether complex or simple) the Coulomb attraction is the dominant force. Even by neutral molecules, intermolecular forces are necessarily electrostatic in origin. By polar molecules the force among permanent electric dipoles is the dominant one. While polarity is not present the force arises from the interaction among instantaneous (fluctuating) dipoles and is known according to the London dispersion or van der Waals' force. Its strength is associated to the polarizability of the molecules concerned. Polarizability usually increases with the size of atoms and the sequence of boiling points He<Ne<Ar<Kr displayed in Fig. 1 reflects this. The boiling point get increases down the group in most series of nonpolar molecules, for instance, CH4<SiH4<GeH4, the diatomic halogens F2<Cl2<... and the order CF4<CCl4<CBr4<CI4 found with other molecular halides. (Ionic halides tend to display the opposite order, reflecting the decrease in lattice energy supposed as the size of ions increases;)
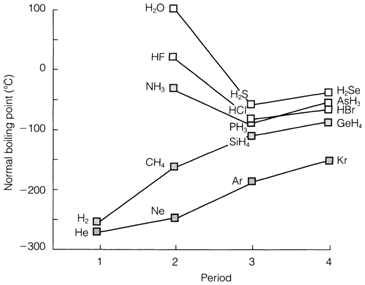
Additionally to forces of a strictly nonbonding nature, molecules might have chemical interactions that contribute to the noticeable intermolecular forces. Acceptor and Donor centers on dissimilar parts of a molecule can lead to self-association and polymerization,. Hydrogen bonding is one demonstration of this kind of interaction, which is particularly significant in polar hydrides of period 2 elements like, NH3, H2O and HF. The extent to which the boiling points of these compounds are out of line as a effects can be seen in Fig. Hydrogen bonding can also have an significant affect on the structure of liquids and solids: So ice has structures in which each water molecule is hydrogen bonded to four others.