Extraction behaviour:
Figures exhibit the extraction behaviour of Zn (II), Cd (II) and Hg (II) in various high molecular weight amines as a function of HCl concentration. In order to illustrate the trends and project the extraction efficiency of various amines, Primene JMT, Amberlite LA - 1 and Amberlite LA - 2, Alamine 336 and tribenzylamine and Aliquat 336 have been included for discussion as representatives of primary, secondary and tertiary amines and quaternary ammonium salt, respectively. In the case of Zn (II) and Cd (II), the behaviour in two diluents namely chloroform and benzene and that of Hg (II) only in chloroform are shown. The curve for tribenzylamine diluted in benzene in not shown because of formation of a precipitate at all molarities of the acid. The curve for Zn (II) in Primene JMT is not shown because of less than 1% extraction over the entire range of acidity.
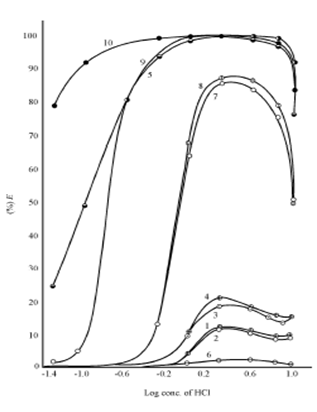
Figure: Extraction of Zn(II)from HCl solutions by various 0.1 M amines in chloroform and benzene. Chloroform solvent: curve 1, Amberlite LA-1: curve 2, Amberlite LA-2; curve 3, Tribenzylamine ; curve 4, Alamine 336 ; curve 5, Aliquat 336. Benzene solvent : curve 6, Primene JM-T ; Amberlite LA-2, : curve 7, Amberlite LA-1, : curve 8, Amberlite LA-2 ; curve 9, Alamine 336 ; curve 10, Aliquat 336
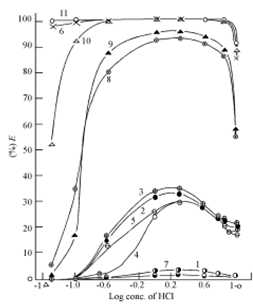
Figure: Extraction of Cd(II) from HCl solutions by various 0.1M amines in chloroform and benzene. Chloroform solvent: curve 1, Primene ; curve 2, Amberlite LA-1 ; curve 3, Amberlite LA-2 ; curve 4, Tribenzylamine, curve 5, Alamine 336 ; curve 6, Aliquat 336 ; Benzene solvent : curve 7, Primene JM-T , curve 8, Amberlite LA-1, curve 9, Amberlite LA-2 ; curve 10, Alamine 336 ; curve 11, Aliquat 336.