Variation of molar conductivity on dilution:
In the case of weak electrolytes (for example CH3COOH, ammonia, organic fatty acids, etc), the ionization will increase with dilution, and hence, the molar conductivity increases with dilution. Therefore the conductivity is directly proportional to the degree of dissociation of a weak electrolyte. These above results are depicted in Figure in which the molar conductivity, Λm, of two electrolytes (KCl and acetic acid) at a constant temperature is plotted against √c. It may be seen from the figure that two different types of behaviours are exhibited by these electrolytes. The strong electrolyte, KCl shows a linear plot (almost straight lines). Instead, the weak electrolyte, CH3COOH seems to approach the dilute solution limit almost tangentially. It is, however, impossible to draw a sharp line of demarcation between the two categories as many substances are known to exhibit intermediate behaviour, example for nickel sulphate. Like electrolytes are sometimes called moderately strong electrolytes.
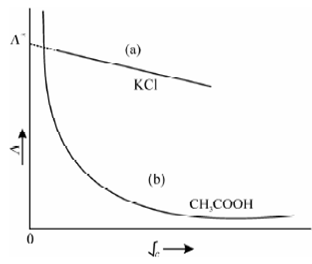
Figure: Variation of molar conductivity on dilution (a) for aqueous solution of potassium chloride (strong electrolyte) (b) acetic acid (weak electrolyte)