Molar conductivity of electrolytes:
Table shows the variation of molar conductivity of a number of electrolytes at various concentrations at 298 K.
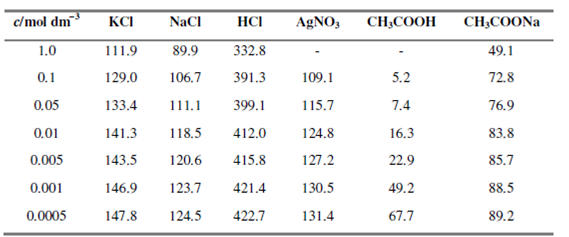
Table : Molar conductivity (104 × S m2 mol-1) of electrolytes in aqueous solutions at 298 K
It is observed that in contrast to the conductivity, the molar conductivit y, Λm invariably increases with decreasing concentration for both strong and weak electrolytes. If we plot a molar conductivities of a huge number of electrolytes against the square root of the concentrations we find that these fall into two distinct categories. In the case of strong electrolytes (for example KCl, NaCl or acids such as HCl, H2SO4, etc), there is a small increase in molar conductivities with the decrease in concentration. Because these electrolytes dissociate almost fully even in concentrated solution, a number of ions do not change much along with concentration. The conductivity should not vary much since it is directly related to the number of ions present in solution. The little changes observed are due to interionic interactions.