Ionic species:
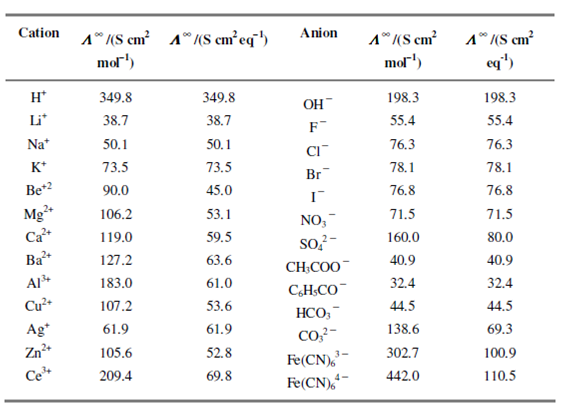
The molar conductivity of the ionic species is a measure of the amount of current carried by ions in question. The Comparison of the molar conductivities of ions is, thus, more useful while associated to per unit charge, for example when Λ ∞ (Na+) is compared with ½Λ ∞ (Mg2+) rather than Λ ∞ (Mg2+) or in terms equivalent conductivities. A value of the limiting ionic molar and equivalent conductivities for some ions in water at 25oC is given in Table 4.2.
Table: Limiting ionic molar conductivities and limiting ionic equivalent conductivities of selected ions in water at 25oC
Ionic Mobilities and Transport Number
The next question that arises in connection to the values of conductivity, given in Table 4.2, is why should there be a difference between the values of limiting molar conductivities of same charged ions, if these ions are only acting like carriers of electric charges only?