Potential of cell:
The potential of cell is given by
Ecell = ESCE - (0.0591log a F- E AgCl +Easy + Ej )
Ecell = E * - 0.0591log a F-
where E * includes EAgCl ,ESCE, Easy and Ej constant potential representing internal reference electrode, asymmetry potential, external reference electrode, and liquid junction, correspondingly. Calibration along with the known fluoride activity eliminates the required of knowing these constants. Fluoride electrode has several applications such as fluoride denoted in bone or air and stack gas samples, chromium plating minerals, baths, water, and toothpastes.
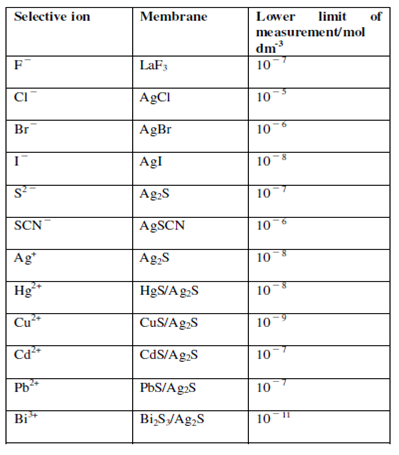
Similar to fluoride ion selective electrode several other solid state membrane electrodes could be developed. Within Table 3.1, we are listing few like ion selective electrodes.
Table 1: Some example of solid state membrane ion selective electrodes
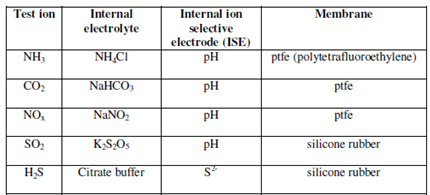
Based on the principle of ion selective electrodes many gas sensing electrodes have been developed in past few years. They are available primarily for the measurement of ammonia, carbon dioxide, and nitrogen oxide. This kind of electrode has a gas permeable membrane and an internal buffer solution. A pH of the buffer solution modifies as the gas reacts along with it. The change is detected through a combination pH sensor inside the housing. This kind of electrode does not needs an external reference electrode.
Table: Several typical gas sensing electrodes