Binary solvent:
Binary solvent mixtures are often used in liquid solid column chromatography. There are various benefits in the use of binary solvent mixtures e.g. solvent strength changes continuously along with composition. Another benefit of binary solvents is that solvent viscosity could be kept low.
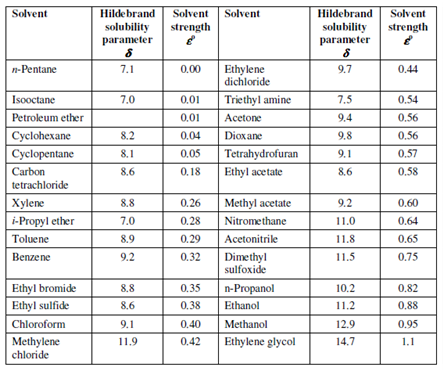
When considering the choice of mobile phases in liquid-liquid column chromatography, a few basic features of mobile phases must be considered. The mobile phase must be immiscible with the stationary phase. It should have viscosity as low as probable for higher column permeability and or efficiency. The detector could also limit the phases that could be used. e.g. strongly UV-absorbing solvents should be prevented along with an ultraviolet photometric detector. The cost, toxicity, purity and stability of a solvent also should be taken into account. Most significant is the selectivity of a liquid-liquid system for a given sample. Within liquid-liquid column chromatography, the k´ values of solutes are commonly controlled through changing the mobile phase. A scale of solvent polarity is described by the Hildebrand solubility parameter, δ. The parameter, δ, is a good measure of what is generally called polarity. Non-polar solvents have low values of δ, while polar solvents have huge values. The values of δ for various solvents are display in Table.
Table : Solvent Strength and Polarity Data