Biocatalysis
Microorganisms can be used as a convenient source of enzymes, since these same proteins provide them with the means to grow on a variety of complex organic compounds. The enzymes can be used in their purified form (either immobilized or in solution) but are sometimes more stable when whole cells are used. Bacterial cells may be rendered nonviable by immobilization or permeabilization but still retains catalytic activity. This can alleviate the need to provide cofactors such as ATP, which can add significantly to costs. The reactions the enzymes catalyze are often regiospecific (attacking a single group on a molecule but leaving others of the same chemical composition) and stereo- specific (attacking one enantiomer such as D-glucose, but not the corresponding stereo- isomer). The specificity of enzymes has allowed industrial chemists to perform reactions that would be impossible by normal synthetic routes, but mostly biotransformations are cheaper to perform and have a higher yield.
Examples of biotransformations are provided in Table 1 but one of the most economically significant biotransformations is the production of acrylamide (a polymer used in many chemical processes as well as in the cosmetics industry). It is possible to use a copper catalyst to convert acrylonitrile into acrylamide, but this reaction must be per- formed at 100?C, after which the catalyst must be regenerated and the unreacted highly toxic acrylonitrile must also be rigorously separated from the product. However, these problems are avoided by the use of immobilized Pseudomonas chlororaphis performing.
Table 1. Examples of industrial production of organic compounds by prokaryotes
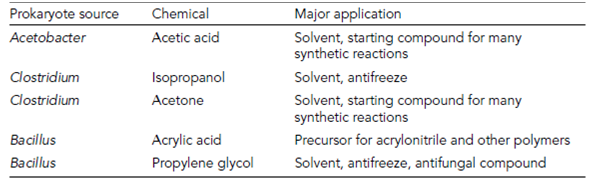
the biotransformation in a bioreactor. The reaction can be run at 10?C, so heating costs are reduced and the bacterial enzyme responsible (nitrile hydratase) converts over 99.9% of the acrylonitrile into acrylamide. Around half a million tonnes of acrylamide are pro- duced annually by this process.
Although biotransformations have been used in the bulk chemical industry, the main application is in the production of fine chemicals such as antibiotic derivatives. The cost savings can be dramatic: cortisone was first synthesized as a 31-step organic synthesis starting from 615 kg of deoxycholic acid. This yielded 1 kg of cortisone, which was sold as an anti-inflammatory drug in the 1940s at around $200 per gram. Use of enzymes from the fungus Aspergillus niger in some of the steps reduced the cost to $6 per gram in 1952. The use of mycobacterial enzymes allowed plant sterols to be used as much cheaper starting compounds, so that by 1980 the price of cortisone had dropped to 46 cents in the United States, about a quarter of 1% of the original cost.