Spectroscopic methods
With the MS, IR and NMR spectroscopies are the most valuable fingerprinting methods for molecular compounds. Characteristics of the spectra also allow structural information to be obtained about a new compound, particularly the existence of known functional groups and some aspects of its symmetry. IR calculates the frequencies of molecular vibrations that depend on the masses of atoms and the force constants (that is the 'stiffness') of chemical bonds. Spectra can be calculated for pure liquid and gaseous samples but solids are generally measured by grinding them to make a mull with a heavy hydrocarbon liquid ('nujol') that has comparatively few and well known, IR bands. Several types of chemical bond, like C-H and C=O, give bands with characteristic IR frequencies and So can be identified. In the example of compound X discussed above, bands appear that are characteristic of the aromatic C-H bonds (suggesting a C6H6 benzene ring) and of C=O groups bound to a metal atom.
In an IR spectrum, the number of bands appearing can frequently give information about the symmetry of a molecule. The method is particularly useful in conjunction with Raman spectroscopy, other way of measuring vibrational frequencies. Raman spectroscopy can also be employed in media like aqueous solution, in which IR measurements are hard or not possible due to the strong absorption by water.
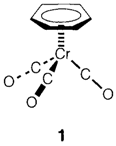
NMR has very distinct principles, depending on the properties of nuclear spin (analogous to electron spin,). All the nuclei not possess spin and of those which do some are much easy to obtain spectra from than others. The known NMR nucleus is 1H; 13C is also helpful for organic and organometallic compounds, and 19F and 31P are specifically easy inorganic nuclei to study, even though many others can be used. Two characteristics of NMR are important. Spectra depict chemical shifts with frequencies for a given nucleus changing with the chemical environment. Spinspin coupling come into existence from the interaction of active nuclei separated by one or more chemical bonds and gives characteristic patterns that allow aspects of the connectivity of atoms to be determined. In compound X, the 1H spectrum depicts that all six hydrogen atoms in the molecule are in a similar chemical environment, with the chemical shift consistent with a benzene ring attached symmetrically to the chromium atom as displayed in 1. 13C NMR confirms two distinct carbon environments with 6 and 3 atoms correspondingly, with chemical shifts suitable to the structure displayed.
One characteristic of NMR can sometimes be misleading. As Compared with most other methods it samples molecules over a relatively long time-scale (usually 0.01 to 0.1 seconds). Some molecules are fluxional with atoms exchanging quickly among different positions, and when this occurs NMR may 'see' these positions as equivalent. For an instance IR and diffraction techniques depict clearly that the PF5 molecule has a trigonal bipyramidal structure with two distinct F positions (equatorial and axial). Though, fluorine atoms exchange so rapidly between these two positions that in 19F NMR all five atoms appear equivalent.
Other spectroscopic techniques can be helpful in some conditions.
Visible/UV absorption spectra rely on the excitation of electrons from filled into empty orbitals. The method has some limited use in fingerprinting but is particularly suited to investigations of electronic structure, particularly the energy variations among molecular orbitals. Topic H8 discusses applications to transition metal complexes as well as the make use of magnetic measurements to determine the number of unpaired electrons.