Osmotic Phenomena:
While a salt solution is separated from pure solvent through a semipermeable membrane, a membrane which allows the passage of solvent but not the solute, it is observed which solvent tends to pass through the membrane within the solution and thus, dilute it. The phenomenon is known as osmosis. Before proceeding, it is essential to define a quantity known as the osmotic pressure (?). For this reasons, consider Figure.
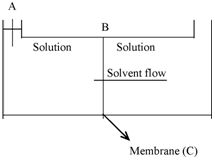
Figure: Osmotic pressure of salt solutions
A is a chamber open at one end and fitted along with a movable piston B. The chamber is divided through means of a semipermeable membrane C, within two sections, of that the right one is filled along with pure solvent, the other with a few salt solution. Because of osmosis, the solvent will tend to pass by the membrane into the solution and displace the piston upward. The motion of the piston and the osmosis could be avoided, therefore, through the application of pressure to the piston in sequence to keep it in the original position. The mechanical pressure which must be applied to prevent osmosis of the solvent into the solution through semipermeable membrane is called osmotic pressure of the solution. This pressure for a provide solution depends on a number of various solution properties. Other than this pressure does not depend on the nature of the membrane so long as the membrane is truly semipermeable.