Multiple membrane-spanning proteins
Various integral proteins have multiple membrane-spanning -helices. Bacteriorhodopsin, a protein establish in a photosynthetic bacterium captures the energy from light and uses it to pump protons across the bacterial membrane. Such as numerous other integral proteins, like as the G protein-coupled receptors and the polypeptide chain of bacteriorhodopsin loops backwards, forwards across the lipid bilayer seven times. Every of the seven transmembrane -helices is associated to the next through a short hydrophilic region of the polypeptide chain which is exposed either on the cytosolic or extracellular side of the membrane. Another multiple membrane spanning proteins have from two to up to 14 transmembrane helices. For instance, the anion exchange band 3 protein of
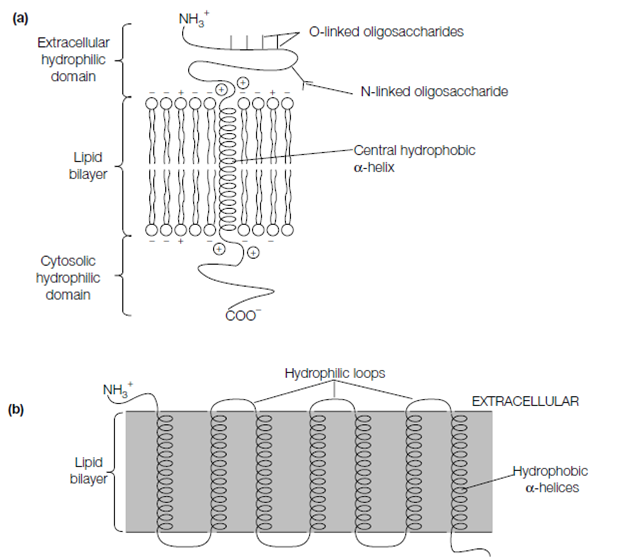
Figure: Integral membrane proteins. (a) Single membrane-spanning region (e.g. glycophorin); (b) multiple membrane-spanning regions (e.g. bacteriorhodopsin).
the erythrocyte plasma membrane which transports HCO– and Cl– across the membrane loops forwards and backwards across the lipid bilayer up to 14 times.