Lipid-anchored proteins
A large number of integral proteins in eukaryotes do not traverse the membrane but are anchored in one or other leafiet of the bilayer through covalent attachment to a hydrocarbon chain. Various proteins, involving the prion protein the causative agent of mad cow disease are stably anchored at the cell surface through covalent linkage of their C-terminal amino acid to the headgroup of a phosphatidylinositol lipid through an ethanolamine–phosphate–trimannose–glucosamine bridge, so-called GPI (glycosyl phosphatidylinositol)-anchored proteins. This complex structure is built up by sequential addition of the individual sugar residues and ethanolamine phosphate to phosphatidylinositol. The C-terminal hydrophobic signal peptide is erased from the protein in the lumen of the RER and the preformed GPI anchor added to the latest exposed C- terminal amino acid.
Another proteins are transiently attached to the cytosolic face of the membrane either through amide linkage of a myristate C14:0 molecule to an N-terminal Gly residue or through thioether linkage of a 15-carbon farnesyl or a 20-carbon geranylgeranyl polyunsaturated hydrocarbon to a C- terminal Cys residue (prenylated proteins). Farnesyl and geranylgeranyl are synthesized from isopentenyl pyrophosphate and the precursor of cholesterol
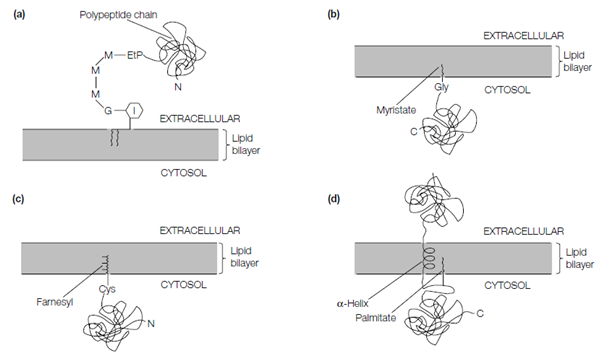
Figure: Lipid-modified proteins. (a) A glycosyl phosphatidylinositol-anchored protein (G, glucosamine; M, mannose; EtP, ethanolamine phosphate); (b) a myristoylated protein; (c) a prenylated protein; (d) a palmitoylated protein.
(See Topic K5). Some proteins are also modified on Cys residues with covalently attached palmitate (C16:0) (palmitoylated proteins). These include some with membrane-spanning polypeptides (Fig. 2d), some prenylated proteins and some myristoylated proteins. Several of the proteins included in cell signaling, like as the G proteins and the Ras family of proteins are lipid modified.