Resting potentials
The resting potential (Vm) occurs as there is a difference in the concentrations of ions between the inside and outside of the cell as shown in table below and as the cell membrane has various permeabilities for these ions. The extracellular fluid is an aqueous solution of sodium chloride. By the contrast, intracellular fluid is an aqueous solution of potassium ions balanced by a variety of anions — involving the phosphates, organic acids, sulfates, a few amino acids, and several proteins—to which the cell membrane is entirely impermeable. The cell membrane is permeable to K+ and as there is a concentration gradient for K+ across the membrane there is a diffusional force acting to drive the K+ from the inside to the outside of the cell as shown in figure. Thus, the cell membrane is entirely impermeable to the much larger anions which therefore remain inside the cell. Since the potassium diffuses out a potential difference forms around the membrane as some of the intracellular anions are no longer neutralized by K+. Now the potential difference means that an attractive electrostatic force is generated which acts to save potassium ions diffusing out. At several point the diffusional force driving K+ out is exactly balanced by the electrostatic force preventing K+leaving.
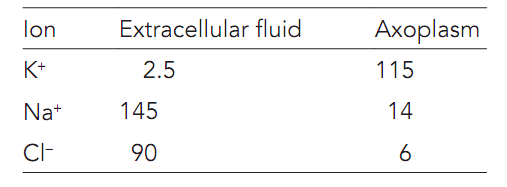
Table: Ionic concentrations across mammalian membranes (mmol-1)
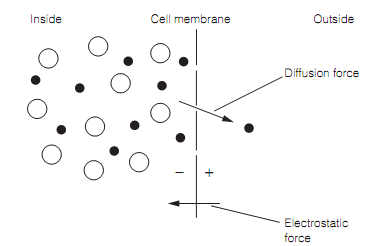
Figure: Illustration of how potassium equilibrium potential is produced. A small potential exists across the membrane whenever the diffusional force equals the electrostatic force. The small filled circles stand for K+ ions; large open circles stand for anions.
At this equilibrium a small potential difference exists, known equilibrium potential since at this potential there is no net flow of K+ ions across the membrane. In the situation where the potential begins as a result of the distribution of diffusible K+ it is termed as potassium equilibrium potential (EK). Usually nerve cells have potassium equilibrium potentials about -90 mV. The three main important points must be noted:
? The transmembrane potentials are all the time quoted as inside relative to the outside, that is taken to be zero. Therefore, EK = -90 mV means that the inside of the cell is negative with respect to the outside.
? The number of ions that migrate across the membrane to begin an equilibrium potential is extremely small.
? The potential difference exists only at the plasma membrane, that by storing charge acts as a capacitor.
The Equilibrium potentials can be computed by using the Nernst equation as follows:
E = (RT/zF) ln Ce/Ci
Here T is temperature in Kelvin, R is the universal gas constant, z is the oxidation state of the ion, F is Faraday’s number and Ce, Ci are the extracellular and intracellular concentrations of the ion respectively.
The potassium equilibrium potential is very close to the resting potential (Vm), for excitable cells advises that Vm arises largely as a outcome of the distribution of potassium I across the cell membrane. The Neuron resting potentials range between -60 mV and -90 mV. The discrepancy between EK and Vm arises as ions other than potassium also make a contribution by virtue of their equilibrium potentials. The main important is sodium (ENa = +55 mV), but as the permeability of Na+ is low as compared to K+ it exerts on a modest influence. The effect of sodium is to drag the resting potential away from Ek towards ENa by an amount which reflects the relative permeabilities of the two ions. The difference among the resting potential and the equilibrium potential for any ion is, Vm - Eion is known as the ionic driving force and is a compute of the electrochemical force tending to move the ion across the cell membrane. At rest the driving force for K+ is quite small while that for Na+ is high. The reason which Na+ does not flow into cells at rest is as the permeability for sodium is very low.
In most of the excitable cells the ionic driving force for chloride ions is close to zero (that is Ecl= Vm). This is as Cl- ions are passively distributed around the membrane accord to the resting potential set up by the combined effects of EK and ENa. The Chloride is passively distributed while K+ and Na+ directly establish the resting potential as the resting concentration gradients for potassium & sodium are actively maintained by the actions of Na+/K+-ATPase, while there are no active transport mechanisms to maintain a fixed chloride concentration gradient.Given that the extracellular and intracellular concentrations of K+, Na+, and Cl-, and the restive permeabilities of these ions, it is very easy to compute the resting potentials. Permeability of cell the membranes to ions is conferred by protein ion channels term leak channels. The family of 15 potassium leak channels (K2P) has been specified.