Lipid bilayer
Amphipathic or amphiphilic molecules hold both hydrophobic (water-hating) regions and hydrophilic or waterloving. The Membrane lipids are amphipathic molecules as they are made up of hydrophobic fatty acid chains and a hydrophilic polar headgroup. In the glycerophospholipids two hydrocarbon chains are hydrophobic while the phosphorylated headgroup and the glycerol backbone are hydrophilic. With In the sphingolipids the fatty acid chain and the hydrocarbon chain of the sphingosine are hydrophobic while the phosphorylated or sugar headgroup is hydrophilic. In the case of cholesterol the whole molecule apart from the hydroxyl group on carbon-3 is hydrophobic in nature.
In the aqueous solution amphipathic molecules will orientate themselves in such like a way as to stop the hydrophobic region coming into contact with the water molecules. In the case of those fatty acid salts that hold only one fatty acid chain like as sodium palmitate, a constituent of soap the molecules form a spherical micellar structure is diameter usually < 20 nm in that the hydrophilic headgroups interact with the surrounding water molecules and the hydrophobic fatty acid chains are hidden inside the micelle. Because the two fatty acid chains of phospholipids are too bulky to fit into the interior of a micelle the special structure for most phospholipids in aqueous solution is a 2D bimolecular sheet or lipid bilayer. Like lipid bilayers, in that the phospholipid molecules are orientated with their hydrophobic chains in the interior of the structure and their hydrophilic headgroups on the surfaces, can be relatively huge structures of up to about 1 mm2 in area. In the two layers of lipids in the bilayer are defined to as the inner, outer leafiets. In biological membranes the individual lipid species are asymmetrically
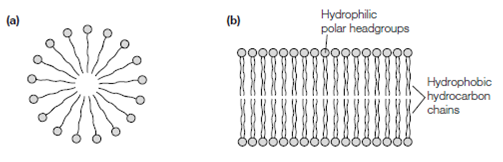
Figure: Structure of (a) a micelle and (b) a lipid bilayer.
distributed among the two leafiets. For instance, in the plasma membrane of phosphatidylcholine, erythrocytes, and sphingomyelin are preferentially situated in the outer leafiet, although phosphatidylserine and phosphatidylethanolamine are commonly in the inner leafiet.The Lipid bilayers will spontaneously self-assemble in aqueous solution. The main driving force following this is the hydrophobic effect the hydrophobic fatty acid chains prevent coming into contact with the water molecules. Once it is formed, the bilayer structure is maintained through multiple noncovalent interactions involving hydrophobic interactions and van der Waals forces among the hydrocarbon chains hydrogen bonding and charge interactions among the polar headgroups, and hydrogen bonding among the headgroups and the surrounding water molecules.