Half curly arrows:
Sometimes reactions take place that involve the movement of single electrons rather than pairs of electrons. Such types of reactions are termed as radical reactions. For instance, a chlorine molecule can be break down into two chlorine radicals on treatment along with light. One of the original bonding electrons ends up on one chlorine radical and the second bonding electron ends up on the another chlorine radical. The movement of these single electrons can be demonstrated through using half curly arrows in place of full curly arrows.
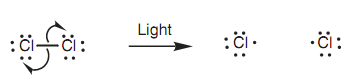
Figure: Use of half curly arrows in a mechanism (homolytic cleavage).
This type of bond breaking is termed as a homolytic cleavage. The radical atoms acquired are neutral but highly reactive species because they have an unpaired valence electron.
There are a number of significant radical reactions in organic chemistry, but the majority of organic reactions include the heterolytic cleavage of covalent bonds in which electrons move together like a pair.
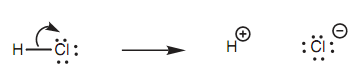
Figure: Heterolytic cleavage of a bond.