Ethanoic acid:
The mechanism that is shown in the figure describes what happens when a hydroxide ion reacts along with a carboxylic acid and is an illustration of how arrows should be drawn. One of the lone pairs of electrons on the hydroxide ion is employed to form a bond to the acidic proton of the carboxylic acid. The curly arrow presenting this begins from a lone pair of electrons and points to the space among the two atoms to depict that a bond is being created.
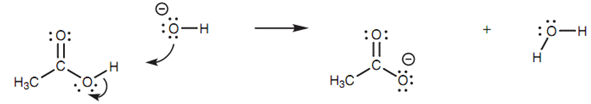
Figure: Mechanism for the reaction of a hydroxide ion with ethanoic acid.
At similar time as this new bond is being created, the O-H bond of the carboxylic acid has to break. This is since the hydrogen atom is only permitted one bond. The electrons in this bond end up on the carboxylate oxygen like a 3rd lone pair of electrons. The arrow presenting this begins from the center of the bond being broken and points directly to the atom in which the electrons will end up as a lone pair.
Observe also what takes place to the charges. The negatively charged oxygen of the hydroxide ion ends up like neutral oxygen in water. This is since one of the lone pairs of oxygen is employed to form the new bond. Both of the electrons are now shared among two atoms and thus the oxygen successfully loses one electron and its negative charge. The oxygen in the carboxylate ion (that was originally neutral in the carboxylic acid) becomes negatively charged because it now has three lone pairs of electrons and has successfully gained an extra electron.