Stereochemical aspects of hydroboration:
The mechanism of oxidation includes addition of the hydroperoxide to the electron deficient boron to create an unstable intermediate that then rearranges like that an alkyl group migrates from the boron atom to the neighboring oxygen and expels a hydroxide ion. After that this procedure is repeated for the remaining two hydrogens on boron and the final trialkyl borate B (OR)3 can then be hydrolyzed with water to provide three molecules of alcohol plus a borate ion.
The mechanism of oxidation occurs with retention of stereochemistry at the alcohol's carbon atom and thus the entire reaction is stereospecific.
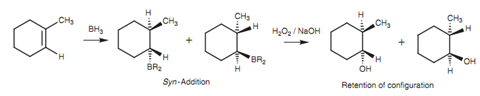
Figure: Stereochemical aspects of hydroboration.
Note: The reaction is stereospecific like that the alcohol group is Trans to the methyl group in the product. Though, it is not enantiospecific and both enantiomers are acquired in equal amounts (a racemate).