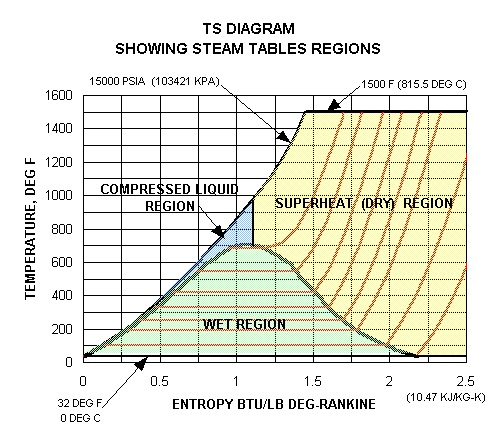
Property Diagrams and Steam Tables
The Property diagrams and steam tables are employed in studying the theoretical and real properties and efficiencies of a particular system.
The phases of a substance and the associations among its properties are most generally shown on property diagrams. A large number of various properties have been defined, and there are some dependencies among properties. For illustration, at standard atmospheric pressure and temperature above 212°F, water exists as steam not a liquid; it exists as a liquid at temperatures between the range of 32°F and 212°F; and, it exists as ice at temperatures below 32°F.
There are five fundamental properties of a substance which are generally shown on property diagrams. These are: temperature (T), pressure (P), specific volume (ν), specific enthalpy (h), and specific entropy (s). Whenever a mixture of two phases, like water and steam, is included, a sixth property, quality (x), is also employed.
The Steam tables contains two set of tables of the energy transfer property of water and steam saturated steam tables and superheated steam tables.