First Law of Thermodynamics
The First Law of Thermodynamics defined as: The energy can neither be created nor destroyed, simply modified in form.
For any system, energy transfer is related with mass and energy cross the control border, exterior work and/or heat crossing the boundary, and alters of stored energy inside the control volume. The mass flow of fluid is related with the potential, kinetic, internal, and "flow" energies which affect the overall energy balance of the system. The swap of external work and/or heat done the energy balance.
The First Law of Thermodynamics is termed to as the Conservation of Energy principle, it means that energy can neither be created nor destroyed, though instead transformed into different forms as the fluid inside the control volume is being considered. The energy balance verbal of here is maintained inside the system being study. The system is an area in space (i.e., control volume) via which the fluid passes. The different energies related with the fluid are then noticed as they cross the boundaries of the system and the balance is prepared.
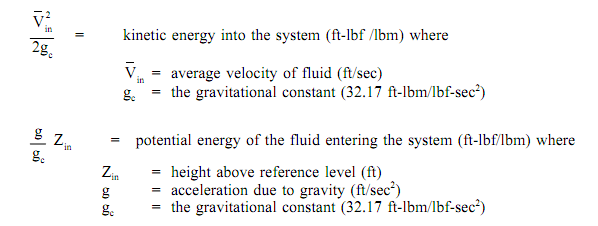
A system might be one of three kinds: isolated, closed, and open. The open system, the most common of the three, points out that mass, heat, & external works are permitted to cross the control boundary. The balance is stated in words as: all energies into the system are equivalent to all energies leaving the system bonus the change in storage of energies inside the system. Remember that energy in thermodynamic systems is comprised of potential energy (PE), kinetic energy (KE), internal energy (U), and flow energy (PL); and also heat and work procedures.
Σ (all energies in) = Σ (all energies out) + Δ(energy stored in system)
Σ Ein = Σ Eout + ΔE storage
For most of the industrial plant applications which most often use cycles, there is no change in storage (that is heat exchangers do not swell whereas in operation). In equation kind, the balance emerges as indicated on the figure shown below.
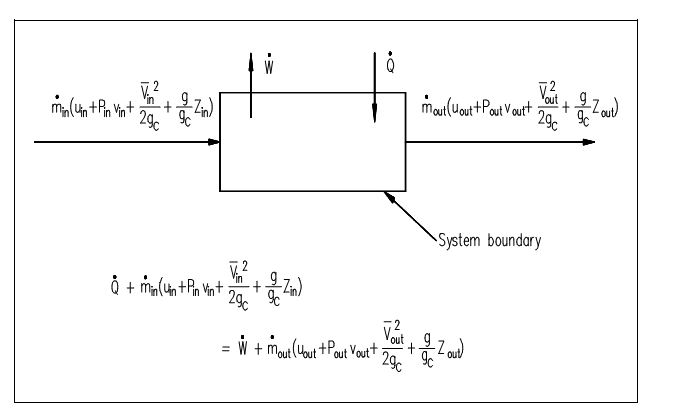
Figure: First Law of Thermodynamics
Here:
Q = heat flow into the system (Btu/hr).
min = mass flow rate into the system (lbm/hr).
uin = specific internal energy into the system (Btu/lbm).
Pinνin = pressure-specific volume energy into the system (ft-lbf/lbm).
W = work flow out of the system (ft-lbf/hr) ?
mout = mass flow rate out of the system (lbm/hr) ?
uou = specific internal energy out of the system (Btu/lbm)
Pout νout = pressure-specific volume energy out of the system (ft-lbf/lbm)

The Heat and/or work can be directed into or out of the control volume. Though, for ease and as a standard convention, the total energy exchange is represented here with the total heat exchange supposed to be into the system and the total work supposed to be out of the system. When no mass crosses the boundary, though work and/or heat do, then the system is termed to as a "closed" system. When mass, work & heat do not cross the boundary (i.e., the only energy exchanges occur are inside the system), then the system is termed to as an isolated system. The Isolated and closed systems are nothing more than particular situation of the open system. In this content, the open system approach to the First Law of Thermodynamics will be highlight since it is more common. Also, approximately all practical applications of the first law require an open system study.