Change of Phase
The phase change of materials in a system is very significant to thermodynamics. It is likely to design systems to take benefit of the phase changes among solid and liquid or among liquid and vapor to improve the performance of the system.
The phase transition is the transformation of a thermodynamic system from one state or phase of matter to the other. The phase of a thermodynamic system and the states of matter contain uniform physical properties.
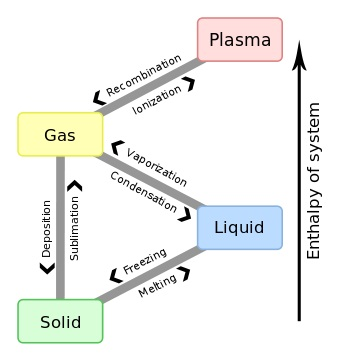
Throughout a phase transition of a given medium some of the properties of medium change, frequently discontinuously, as an outcome of some exterior condition, like pressure, temperature, and others. For illustration, a liquid might become gas upon heating to the boiling point, resultant in a sudden change in volume. The measurement of exterior conditions at which the transformation takes place is termed as the phase transition
The Phase transitions are general occurrences noticed in nature and many engineering methods exploit certain kinds of phase transition.
The term is most generally used to explain transitions among solid, liquid and gaseous states of matter, and, in exceptional cases, plasma.