pH Titration:
Similar to potentiometric titrations, in compare to direct pH measurements, pH titrations generally offer increased accuracy and precision. Accuracy is rise since, measured pH are used to detect rapid changes in activity that occur at equivalence point of the titration. In addition, it is the change in pH versus titre volume rather than absolute value of pH that is of interest. Therefore, the errors because of liquid-junction potentials and activity coefficients are minimized. pH titrations may be applied to a variety of systems including those involving weak acids and weak bases. In such titration, it is difficult to get end point using indicator method. A typical acid- base titration using pH metry is briefed as follows.
It is known that the neutralization of acids and bases is always accompanied by the changes in the concentration of H+ and OH- ions. It is evident that hydrogen electrode may be employed in these titrations. A reference electrode used in these titrations is 1 M-calomel electrode. A apparatus used for acid-base titrations is as shown in Figure.
The critical problem in titration is the recognition of point at which the quantities of reacting species are presented in equivalent amounts, i.e. the equivalence point. The titration curve can be followed point by point plotting as the ordinate successive values of the pH versus the corresponding volume of titrant added as the abscissa. Addition of the titrant should be the smallest accurately measurable increments that gives an adequate density of points, mainly within the vicinity of equivalence point.
- Over most of the titration range the pH varies gradually, but near the end point the pH changes very abruptly. The resulting titration curve resembles Figure (a).
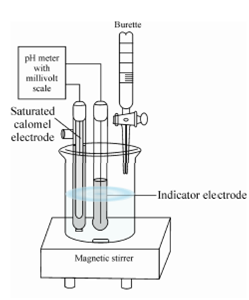
Figure: Typical Instrumental set up for pH titration