Theory of Mass Spectrometry:
Have you ever wondered what would happen if we take a molecule and bombard it with a beam of high energy electrons? Taking clue to the ionisation of atoms to provide ions you might be prompted to say that they would ionise; and you are absolutely right. This is what exactly is done in mass spectrometry. Here the molecule in the vapour phase is bombarded with a beam of high energy (~ 70 eV) electrons. These electrons knock off an electron from the molecule leading to the formation of a positively charged molecular ion, or more appropriately a radical ion or radical cation. For example, the radical ion formation from ammonia can be represented as
NH3 + e- → NH.+3 + 2e-
The radical ions so obtained have a large amount of extra energy which is much more than that required for breaking the covalent bonds. Therefore, the highly energetic molecular ions undergo fragmentation yielding smaller fragments. For instance,
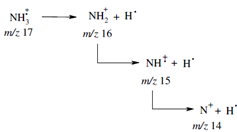
As the molecular ion can fragment in a number of ways depending on the nature of the molecule, the fragments provide clue to the structure of the molecule. The radical ion and the fragments obtained from the molecule are then separated according to their m/ z value (where m is the mass and z is the charge on the ion) using magnetic and/or amounts or intensities is known as mass spectrum of the sample.