Effective nuclear charge
The electrostatic repulsion among negatively charged electrons has a large affect on the energies of orbitals. So, the ionization energy of a neutral helium atom (two electrons) is 24.58 eV compared with the 54.40 eV for that of He+ (one electron). The influence of repulsion is explained as shielding or screening. The combined influence of attraction to the repulsion and nucleus from other electrons gives an effective nuclear charge Zeff, that is less than that (Z) of the 'bare' nucleus. One quantitative definition is from orbital energy ε using the equation:
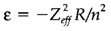
Where R is the Rydberg constant and n is the principal quantum number. For instance, applying this equation to He (n=1) gives Zeff=1.34.
The dissimilarity between the 'bare' and the effective nuclear charge is the screening constant σ:
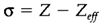
For instance, σ=0.66 in He, showing that the influence of repulsion from one electron on another has an effect equal to the reducing the nuclear charge through 0.66 units.