Associative LTP biochemistry
CA1 cells have NMDA and AMPA glutamate receptors. In order to be trigger NMDA receptors must attach experience and glutamate a depolarization big enough to erase Mg2+ ions from the channel (voltage-dependent blockade). This condition is not provided through low-frequency Schaffer collaterals stimulation. The amount of glutamate released is low few AMPA receptors are activated and the resulting epsp is too small to open NMDA receptors. Moreover high-frequency stimulation opens numerous AMPA receptors and so depolarizes the cell sufficiently to activate NMDA receptors. Cooperativity become because the more afferents activated the greater the depolarization of CA1 cells. The Associativity is property of the NMDA receptor itself: to activate it needs the coincidence of presynaptic glutamate release and postsynaptic neuron depolarization (Hebb’s rule).
It is Ca2+ entry through NMDA receptors that triggers the induction of E-LTP at CA3-CA1 synapses. Antagonists of NMDA receptors prevent the induction of LTP. Crucially for the idea that Long-term potentiation is a cellular substrate of learning, manipulations that impair Long-term potentiation cause learning deficits and vice versa. Preventing NMDA receptors from functioning not only blocks induction of LTP; it also prevents some types of learning. Pharmacological blockade of NMDA receptors impairs learning of the Morris water maze by rats. Mice genetically engineered so that NMDA receptor subunits are deleted from the CA1 region in the third postnatal week (after hippocampal development is complete) show no NMDA- receptor dependent Long-term potentiation in CA1, have impaired spatial learning in the Morris water maze and define a loss of the correlated firing of place cells that is seen in normal animals.
NMDA receptors are needed for induction of Long-term potentiation in most locations in the CNS but at some synapses metabotropic glutamate receptors or kainate receptors are required.
Both postsynaptic and presynaptic sites are implicated in the expression of Long-term potentiation but the specifics depend very much on the kind of Long-term potentiation and the synapses included. At the CA3– CA1 synapses postsynaptic changes include:
- An increase in responsiveness of the postsynaptic membrane as AMPA receptors become more sensitive to glutamate. Calcium–calmodulin dependent protein kinase II CaMKII a main protein of the postsynaptic density, is thought to be activated via Ca2+ entry by NMDA receptors and it then phosphorylates AMPA receptors (GluR1 subunits) enhancing their response to glutamate. Biochemistry of this has been worked out.
- Activation of silent synapses. These are synapses which harbor only NMDA receptors.They are normally silent because low-frequency stimulation does not activate NMDA receptors. During LTP Calcium entry induction causes them to acquire AMPA receptors and so become responsive to low-frequency input.
Presynaptic parts to CA3–CA1 synapse Long-term potentiation have been proposed in which there is increased glutamate release. This requires that the NMDA Ca2+ signal generates a retrograde messenger in the postsynaptic cell which travels the “wrong” way across the synaptic cleft. For this role one molecule proposed is NO which is nitric oxide. In neurons nitric oxide is synthesized by a Ca2+-dependent nitric oxide synthase (NOS). As a tiny freely diffusible molecule, nitric oxide rapidly diffuses out of the postsynaptic cell transversely the cleft and into the presynaptic cell where it stimulates guanylyl cyclase, thus enhancing the probability of glutamate release.
With short tetanic stimulation Long-term potentiation lasts for about 2 hours. Various mechanisms probably have a role in this maintenance of E-LTP. They must explain how synaptic alterations are retained even although individual molecules are being turned over, after the original signals which brought about the alterations have gone. Two well-documented processes are as belows:
- CaMKII consists of four subunits. Ca2+ activation phosphorylates them and once the Ca2+ concentration has fallen to resting stages they remain phosphorylated. It will immediately become autonomously phosphorylated if a subunit becomes dephosphorylated by one of the other subunits. In this path CaMKII will remains persistently active.
- Ca2+ stimulates an isoform of adenylyl cyclase and consequently cAMP concentrations increase in Long-term potentiation. Protein kinase A becomes persistently activated and has effects on gene expression.
A number of kinases are activated in E-LTP but ERK (extracellular signal-related kinase) is thought to be important in the transition to L-LTP which occurs if various tetanic stimuli are delivered. This long-lasting L-LTP is blocked through drugs which inhibit mRNA or protein synthesis which shows that it depends on transcription and translation. In L-LTP morphological as well as functional changes are seen. This involves the changes to the geometry of dendritic spines and the formation of new synapses by the splitting of old ones into two.
Late phase synaptic plasticity depends on synthesis of proteins that must function only in activated synapses; synaptic tagging. The idea is that a specific protein or perhaps a process (altered mRNA translation or cytoskeletal assembly) marks out an activated synapse so that plasticity-related proteins can then assemble to produce the functional and morphological changes that result in persistent synapse strengthening. Synaptic tagging was proposed as a theoretical means to ensure input specificity as long ago as 1997 but only recently has direct evidence for it come to light. NMDA receptor activation has been shown to trigger input-specific spine entry of specific proteins which could serve as synaptic tags.Key events in LTP are summarized diagrammatically in Figure. Somewhat different mechanisms are responsible for nonassociative LTP that occurs at some other synapses.
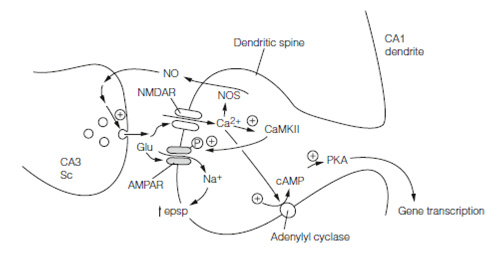
Figure: Key events in LTP. Sc, Schaffer collateral; Glu, glutamate; AMPAR, AMPA receptor; NMDAR, NMDA receptor; NO, nitric oxide; NOS, nitric oxide synthase; PKA, protein kinase A; CaMKII, Ca2+–calmodulin-dependent kinase II.