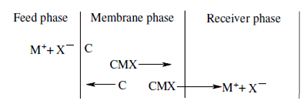
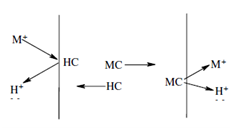
Equilibrium constant:
The equilibrium constant is provided through the expression
Kd = [MC2 ( membrane) ] [H+ (aq)]/[HC(membrane) ]2 [M2+(aq ) ]
Through inverting the concentration terms in Eq. 11.9 we get the equilibrium constant at the receiving side of the membrane. The whole equilibrium constant is achieved although there is no additional metal ion transport from the feed to the receiving side and the following relationship applies.
log [(M2+) receiving phase / (M2+) feed ] = 2 ? pH
where, ? pH is the pH difference among the feed and the strip solution. If for instance , the pH values for the feed and receiving solutions are 4 and 1, correspondingly , M2+ could be concentrated in the receiving solution to as high as 10 6 : 1 associative to its concentration in the feed.