Hydration Shell:
You will see the Li+ ion, because of its larger hydration shell, has a lower mobility than the potassium ion. Similar argument can be applied to the F- & Br- ions. Exceptional mobilities are observed for the H+ and OH- ions. This is because, in these case charge is transported through proton jump mechanism along with general migrations mechanism, consider the case of H+ ion.
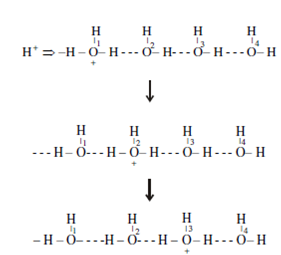
You can see how hydrogen ion jumps from O1 to O2; O2 to O3... this result is equivalent to as the migration of charge from left to right. This conduction mechanism is more like a charge than ion movement. Like conduction is likely since of the peculiar structure of water and therefore just found in hydrogen-bonded solvents.