Other geometries
The pattern of ligand field splitting relies on the coordination geometry; usually those d orbitals that point very strongly towards the ligands are increased in energy comparative to the others. Figure 4 depicts the splittings produced through some other ligand coordination geometries. Tetrahedral (Td) coordination provides a splitting in the reverse direction (and approximately half the magnitude) to that found along with octahedral. Tetragonally distorted octahedral (D4h) coordination come into existence in which two opposite ligands are further from the metal than the other four. In this and in square-planar coordination (also D4h), the d orbital pointing in the direction of ligands in the xy plane is higher in energy than the others. (The main variation from the octahedral case is the lowering in energy of dz2as this interacts less strongly along with the ligands).
Ligand field splitting is occasionally significant in considerate the geometrical preferences of an ion, even though other issues may play a part. The splitting in tetrahedral coordination is just about half that for octahedral, and so in competition among octahedral and tetrahedral geometry the octahedral LFSE is more significant; thus ions like Cr3+ (d3) and Co3+ (d6 low-spin) are almost all the time found in octahedral coordination and are remarkably resistant to creating tetrahedral complexes. Square-planar complexes are found for d8 ions while the ligand field splitting is sufficiently large for the electrons to pair in the four lowest orbitals (see Fig. 4c); instances are Ni2+ with strong-field ligands, and Pd2+ and Pt2+ in almost all circumstances.
The geometry of Fig. 4b takes place from a so-called Jahn-Teller distortion of the octahedron. The eg orbitals are split in energy, and this permits stabilization of a complex if these two orbitals are not equally occupied. So in d9 (Cu2+) two electrons take place the dz2 and Dx2-y2. Almost all Cu2+ compounds depict this type of distortion, as do several high spin d4 ions like Cr2+.
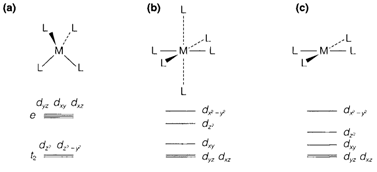
Fig. Ligand field splitting patterns for (a) tetrahedral, (b) tetragonally distorted octahedral, and (c) square-planar complexes.