Polymerization
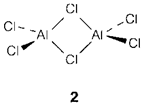
The trend of many molecules to aggregate and form dimers (example Al2Cl6 2), larger oligomers or expanded polymeric structures can be regarded as a donor-acceptor interaction. So in the reaction
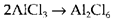
To one AlCl3, a chlorine atom bound uses nonbonding electrons to complex with the other aluminum atom; like in most other instances of this kind of the bridging atoms are symmetrically disposed with the same bonds to each aluminum. Polymerization of AXn molecules is more likely to take place when n is small and when the atom A has vacant orbitals and is sufficiently large to increase its coordination number. Several oxides and halides of stoichiometry AB2 and AB3 form structures that may be considered as polymeric, even though the distinction between this (polar covalent) explanation and an ionic one is not clear-cut.
Hydrogen bonding can also be considered as as a donor-acceptor interaction in which the acceptor LUMO is the (unoccupied) antibonding orbital of hydrogen bonded to an electronegative element.