Lewis bases:
A Lewis base is a molecule that can give a lone pair of electrons to fill the valence shell of a Lewis acid. The base can be a negatively charged group like a halide, or a neutral molecule like water, an amine or ether, as long as there is an atom exist with a lone pair of electrons (that is O, N, or a halogen).
All the Brønsted-Lowry bases that described earlier can also be defined as Lewis bases. The main feature is the existence of a lone pair of electrons that is available for bonding. Hence, all negatively charged ions and all functional groups consisting of a nitrogen, oxygen, or halogen atom can work as Lewis bases.
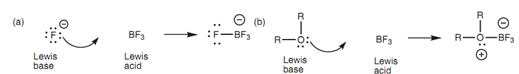
Figure: Reactions between Lewis acids and Lewis bases.