Hard-soft classification
It is established that the comparative strength of donors relies on the nature of the acceptor and vice versa. The hard and soft acid-base (HSAB) classification is frequently employed to rationalize some of the variations. While two acids (A1 and A2) are in competition for two bases (B1 and B2) the equilibrium
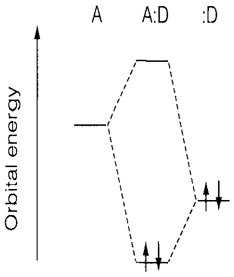
Fig. 1. Molecular orbital interaction between a donor (:D) and an acceptor (A).
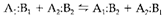
will lie down in the direction in which the harder of the two acids is in combination with the harder base and the softer acid with the softer base. Like a standard for comparison the prototype hard acid H+ and soft acid [(CH3)Hg]+ are frequently used. So the equilibrium will lie to the left side or right as per degree of hardness of the base B.
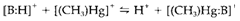
Instances of hard acids are H+, cations of very electropositive metals like Mg2+ and nonmetal fluorides such as BF3. Soft acids that includes cations of late transition and post-transition metals like Cu+, Pd2+ and Hg2+. The hardness of bases get increases with the group number of the donor atom (example NH3<H2O<F-) and get decreases down any group (example NH3>PH3, and F->Cl->Br->I-).
Even though the hard-soft classification provides a helpful systematization of several trends it does not by itself provide an description of the different behavior. Usually it is referred as that hard-hard interactions have a greater electrostatic component and soft-soft ones rely more on orbital interactions but several other issues may be involved. Soft donor and acceptor atoms are frequently large and van der Waals' forces might contribute to the bonding; some soft bases like CO also demonstrate π-acceptor behaviour. It is also significant to remember that hard and soft behaviour is defined in a competitive situation. While reactions are studied in solution some competition with solvation is all the time present.