The Born-Haber cycle
The lattice energy UL of a solid compound is described as the energy required to transform it into gas-phase ions, for an instance,
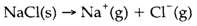
(Note: sometimes the opposite process is employed as a definition that makes UL a negative quantity rather than positive as here.) It is usually assumed that the compound concerned is ionic but a lattice energy can be described without that assumption, provided the ions created in the gas phase are clearly specified.
Lattice energies might be estimated from a thermodynamic cycle termed as a Born-Haber cycle, that makes use of Hess' Law. Strictly telling, the quantities involved are enthalpy rather than energy changes and one should write HL for the lattice enthalpy. By the Fig. 1. that depicts a cycle for NaCl, we see that
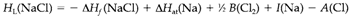
In which the terms on the right side are, in order: the enthalpy of formation of NaCl, the enthalpy of atomization of Na solid, the bond enthalpy of Cl2, the ionization energy of Na and the electron affinity of Cl.
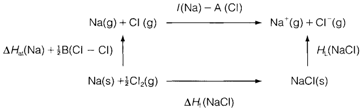
Fig. 1. Born-Haber cycle for determining the lattice enthalpy of NaCl.
While multiply charged ions are involved the cycle can be adapted by summing higher ionization energies or electron affinities as suitable. I(Na) is greater than A(Cl) in the equation above. This depicts that in the gas phase, Cl and Na atoms are more stable than the ions Cl- and Na+ and it is the lattice energy that stabilizes the ionic charge distribution in solid NaCl. A identical result is found for all ionic solids.