Catalytic Currents:
While electroactive substance is decreased at the electrode through normal process
O + ne- = R
and if the solution holds another substance, Z that is not electroactive or reduces at more negative potential than 'O', it might catalyze the back reaction and the reagent O is produced through the following mechanism
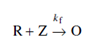
Because of the reproduction of electroactive substance, more and more 'O' is reduced at DME and there is an increase in current that is catalytic current. This process is continued until the applied potential is extremely much negative corresponding to the limiting plateau of 'O'. An average catalytic current over the life of a drop is
Figure: Polarographic Catalytic curve