Adsorption Waves:
Some electroactive species or their products because of electrooxidation or reduction are adsorbed on the surface of mercury drop and provide adsorption waves. In electrode reaction
O + ne- → R
if 'R' is adsorbed, the current obtained is small (adsorption wave) and a single wave is obtained whose height is proportional to 'O'. But after certain concentrations while the surface of the drop is saturated along with 'R', a second wave is observed at more negative potential that is the 'normal wave'. This general wave represents a reduction of 'O' to dissolved 'R'.
From the time the general wave appeared, a height of the adsorption wave remains constant. The total wave of first and second together is proportional to the concentration of 'O' and is diffusion controlled.
If 'O' is adsorbed and not 'R', the common wave appears on the positive side of the adsorption wave.
Adsorption waves are observed within solutions of thiols, many organic uracils, mercurials, arsenic (III) etc.
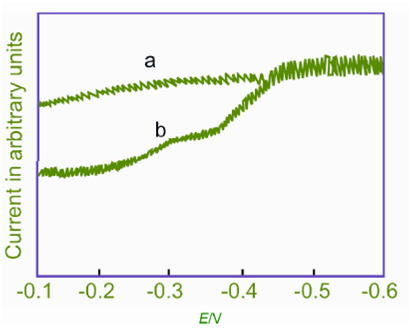
Figure Polarogram of sodium propyl xanthate
(a) 0.2 M NH4Cl, pH 6. 5
(b) a + 1.2 mM sodium propyl xanthate (adsorption + normal wave)
Those adsorption waves have been used to evaluate the area of adsorbed species by using the equation provided below while only adsorption is seen without normal wave.
ia =13.66 nm 2/3 t -1/3 / A
where 'A' is the area (in Å ) covered by each adsorbed molecule.