Base-catalyzed mechanism for keto–enol tautomerism:
This results in the carbonyl oxygen acquiring a positive charge that activates the carbonyl group to attack through weak nucleophiles. The weak nucleophile in question is a water molecule that eliminates the α-proton from the ketone, resultant in the creation of a new C = C double bond and cleavage of the carbonyl π bond. The enol tautomer is created so neutralizing the unfavorable positive charge on the oxygen. With in basic conditions, an enolate ion is created, that after that reacts with water to form the enol.
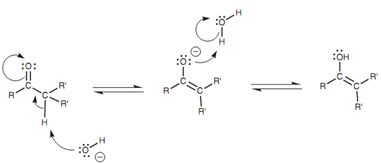
Figure: Base-catalyzed mechanism for keto-enol tautomerism.