Possible electronic spin states of the molecules:
The energies of the two excited spin states are also different because of the difference in the interactions among electrons; the energy of the triplet state commonly being lower than in which of the singlet state. Within the Jablonski diagram two excited singlet states (S1 and S2) and a triplet state (T1) are displays. The absorption and emission of the radiation leads to the activation and deactivation procedures. Let us learn about the way activation (through absorption of radiation) and deactivation processes take place.
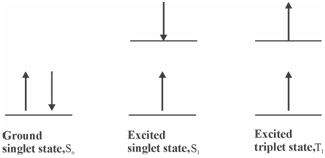
Figure: Possible electronic spin states of the molecules