Relative isotopic abundance:
The other peaks in the spectrum appear due to the fragmentation of the molecular ion. The natural abundance of some common elements is compiled in Table.
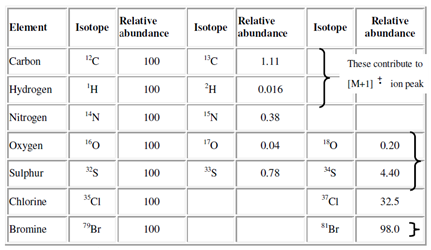
Table: Relative isotopic abundance* of some common elements
The presence and the intensity of peaks at m/z values greater than that of the molecular ion can provide important information about the elemental composition of the molecule. As an instance let us have a look at the mass spectrum given in Figure.
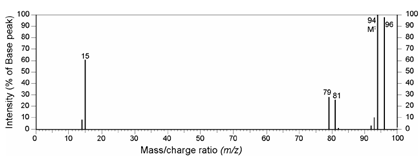
Figure: A mass spectrum showing the importance of isotopic peaks
What do you observe in this case? Have a look at the Table again, what do you infer? Yes it is a typical case of a molecule containing a bromine atom; the intensities of M and [M+2] peaks are comparable. The spectrum is of a simple molecule, bromomethane, CH3Br.