Schematic illustration:
A schematic illustration of various steps in IDA determination is shown in Figure. This procedure is also called direct isotope dilution analysis (DIDA). In an alternate method called inverse isotope dilution analysis (IIDA) amount of radioactive substance (m*) in the sample solution with activity A1 can be determined by adding inactive component (m1) and some fraction of the pure component having activity A2 is isolated. In this case mass of radioactive substance (m*) is determined using the equation:
m* = (A1/A2)m - m1
while, m and A2 are the weight of recovered sample and its activity correspondingly. Therefore quantity of the radioactive substance initially present in the sample or its specific activity is calculated. It has the benefits in which quantity of exact radioactive component of a sample without comparing it with a known radioactive standard can be determined. This procedure is especially useful for determining the amount of radionuclide present in a complex mixture of radionuclides, say spent nuclear fuel or reactor irradiated sample.
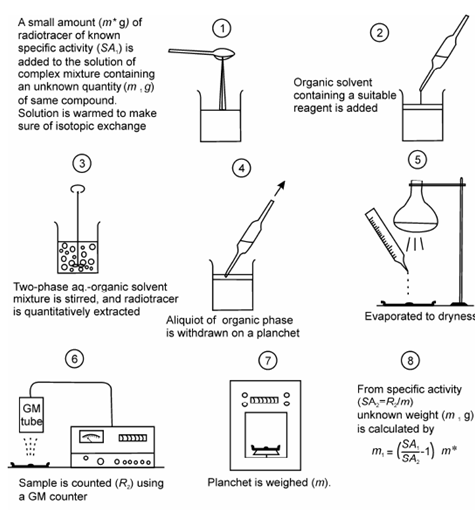
Figure: Schematic illustration of sequence of steps for the determination of an element or a compound in a complex mixture by IDA