Explanation of Reading Iron-Carbon Phase Diagram
Assume that in liquid state mixture of iron and carbon contains 0.4% carbon and is represented by point 1 in Figure 8. The line xx shows the path of cooling.
As cooling begins the freezing starts in intersection point 2 of xx and liquidus ABC at 1510oC. At a temperature of 1470oC at point 3 the liquid is solidified completely into solid austenite. This phase slowly cools until point 4 on line IK is reached. The precipitation of proeutectoid ferrite begins and the carbon content of austenite varies along with line A3. The composition of ferrite varies along with the line IL. In the region enclosed among IL and IK his percentage of austenite and ferrite can be determined by utilizing lever rule. The remaining austenite transforms into peralite (88% ferrite and 12% cementite) at eutectoid temperature of 723oC. The % weight of (ferrite + cementite) might be determined by lever rule along line LKM. Further cooling shall effect no change in microstructure since carbon percentage in ferrite practically remains constant of 0.008. The microstructure is made by pearlite matrix embedded with ferrite crystals.
Taking example of alloys with 3% carbon and considering its cooling from a point above liquids along yy. Of course the composition is in the region of cast iron. The austenite separates from liquid and its content will increase along the solidus PG and the amount of liquid reduces with composition varying along liquidus BC. While the temperature of 1130C is reached, the mixture shall consist of austenite containing 2% carbon and liquid of eutectic composition.
The liquid will solidify at constant temperature into ledeburite – a mixture of austenite and cementite. On added cooling eutectic austenite decomposes to cementite along with the line GK. At temperature of 723oC (eutectoid). The remaining austenite shall transform to pearlite. Below the temperature of 723oC all of ledeburite shall be transformed into a mixture of cementite and pearlite.
Transformation Reactions
Several transformations were described in above paragraphs whereas explaining how to read iron-carbon diagram. These transformations are summed up afresh here.
Eutectoid Reaction
Eutectoid reaction takes place while austenite containing 0.77% C decomposes into ferrite and cementite at 723oC (point K in the phase diagram of Figure ).
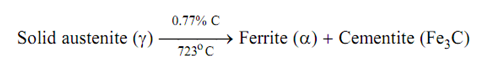
Solid phase splits into two solids phases. Steel containing carbon among 0.008 and 0.8% is known as hypoeutectoid and those containing among 0.8 and 2.0% carbon are hypereutectoid.
Eutectic Reaction
Eutectic reaction occurs when liquid solution containing 4.3% C transformations into austenite (γ) and cementite (Fe3C) at 1130oC. Point C in Figure is eutectic point.
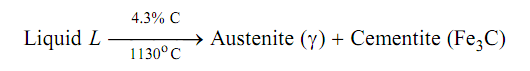
Liquid phase transform in two solid phases.
Figure : Iron-Carbon Phase Diagram