Time Temperature Transformations
The phase diagram of iron-carbon system evokes much interest for engineers to use the information for useful purposes of increasing strength and eliminating locked in stresses by retaining or avoiding a particular phase. Though, significant aspect of such a diagram to keep in mind is that it represents equilibrium cooling. Such equilibrium cooling is neither obtained in practice nor is it conducive to develop desired strength and structure in particular steel. Increasing cooling rates decrease the transformation temperature as was highlighted and may result information of metastable phase. As an example very high cooling rate of iron-carbon system in steel range causes development of a metastable phase called martensite. This phase doesn’t appear in Figure 8. Normally the steel and cast iron carry some alloying elements, although in varying amounts. These alloying elements have great deal of influence on precipitation of all the phases and this is also not represented by phase diagram of Figure .
The metastable phase marteniste has been introduced here and other phases like pearlite ferrite, and cementite were mentioned earlier. More regarding their structure and properties will be discussed now but before that transformation curves are described.
Experimental determination of isothermal transformation curves goes as under. A number of samples of the size of a 50 paise coin are austenised just above the temperature of 723oC (eutectoid temperature). The samples are quickly cooled in a salt bath maintained at a temperature slightly below 723oC. After allowing several time intervals the specimens are taken out one by one from the salt both and quenched into water at room temperature. The resulting microstructure is then examined at room temperature. The experiment is repeated with isothermal transformation of eutectoid steel (both regions of hypo- and hypereutectoid steel) at progressively reducing temperatures. If temperature of transformation is plotted as function of time of transformation the resulting curve as shown schematically in Figure 9 is called isothermal transformation (IT) curve, or temperature-time-transformation curve (TTT). This is also known as Bain curve after the metallurgist who first introduced the idea of S curve because of its shape.
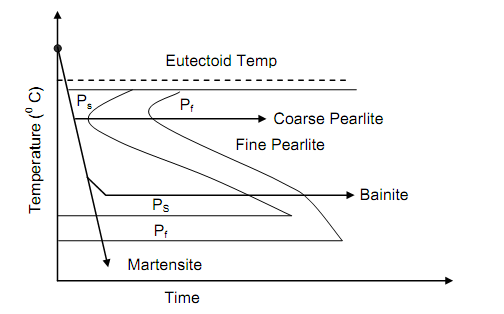
Figure: Time Temperature Transformation Diagram for Plain Carbon Steel
The line marked Ps shows the beginning of transformation of austenite into pearlite and the line Pf represents the completion of such transformation. Figures show the TTT diagrams respectively for 0.8% C steel and 0.3% steel. The difference between the two can be noted. Even the fastest cooling rate shall not be able to mess the nose of the S curve and hence 100% martensite will not be retained at room temperature in structure of steel. The significant feature of TTT curve is its bending backwards at nose. Below the nose the austenite transforms into Bainite. Whether this is pearlite or bainite, at a temperature called Ms the transformation into a transition phase Martenstite occur.
Both binite and martensite might be retained in steel by controlling the rate of cooling. If cooling is adequately fast so that nose is avoided then austenite transforms into bainite. If the part is now held at this temperature for sufficiently long time the bainite is stabilised. Unlike, pearlite, in bainite the cementite is in particle form, distributed uniformly in the matrix of ferrite. Bainite is stronger, harder & tougher than pearlite. Bainitic steel is more ductile than pearlitic steel for some level of hardness.
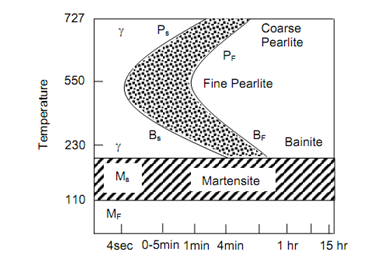
Figure: 0.8% C Steel
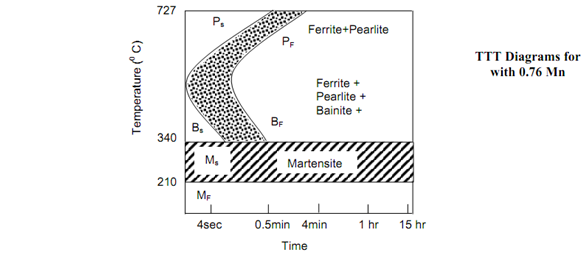
Figure : TTT Diagrams for 0.3% C Hypoeutectoid Steel
If heated steel is cooled sufficiently fast the nose of Ms temperature, martensite is formed. Its transformation is complete at temperature Mf. Martensite has C dissolved in Fe whereby its bcc structure changes into body centered tetragonal (bct) structure and is marked by high hardness because of
1. distortion of iron lattice,
2. very fine size of martensite plates, and
3. high density of dislocations linked with twining.
Figure schematically shows the cooling pattern to produce different phases in steel. Heat treatments given to steel will be governed by the cooling rates producing final phases with pearlite, bainite or martensite. Several refinements are possible with control of cooling rate and soaking time.
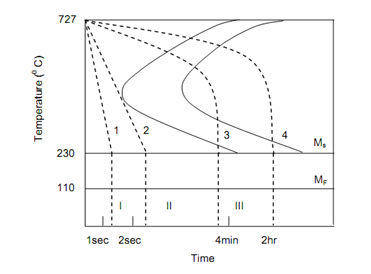
TTT Curves for Steel and Different Cooling Rates
At this stage, before taking different methods of heat treatments in any detail, it shall be worthwhile to first describe formation and characteristics of different phases.