Mechanism:
The mechanism could be explained on the following lines.
i) The addition of organic content reduces the dielectric constant of the solution promoting thereby ion pair formation among the ionic species and fixed ions of the ion exchange. Therefore, with increasing organic content, the uptake of ionic species on the ion exchanger may increase.
ii) Sometimes, the addition of organic content of the aqueous solution causes a decrease in the distribution coefficient of the ion. This is probably due to the attachment of the anionic complex to the protonated organic solvent making thereby the anionic complex less available for the solid anion exchanger. It may be worthwhile to explain the formation of this ion-pair between the protonated organic solvent and the anionic metal complex on the lines. That mechanism in this case can be illustrated as follows:
(Organic solvent) + H+ ↔ ( Protonated organic solvent)
(Protonated organic solvent) + ( Anionic complex of the metal)
?
{(Protonated organic solvent) ( Anionic complex of the metal)}/ Ion association complex
It may be important to point out that the force of attraction between the two ionic components of the ion association complex increases with the increasing organic content that is, decreasing dielectric constant.
iii) In a few rare cases, the organic solvent may form complexes with the metal ions bringing a change in their distribution coefficient values.
The ion exchange and solvent extraction may operate simultaneously in a particular system and compete with each other resulting in to some irregular trends. Sometimes, the irregularity of these trends is difficult to explain. Nevertheless, it does not reduce the potentiality of the technique for separation purposes. In order to highlight the utility of the technique, the distribution coefficient of Au (III) and Hg(II) for Amberlite IR - 400 (anion exchanger) at 0.6 M HCl with changing percentage of tetrahydrofuran (THF) are given below in Table.
Table: I Values Data
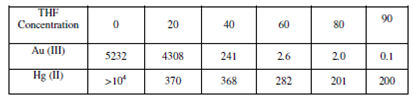
In Table, there is a decreasing trend in the distribution coefficients of both the metal ions with the increasing percentage of THF content. Without any THF at 0.6 M HCl, both Au (III) and Hg (II) are strongly adsorbed on Amberlite IR-400 and it is difficult to separate them. The best condition for separation is achieved at 0.6 M HCl with 90% THF.