Microporosity:
During preparation of ion exchanger through silylation, a vinyl group is chosen for R3 in -SiOSiR1R2R3 leading to a vinylated silica which is then polymerized with styrene.
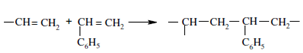
Afterwards, the bonded phase is treated with chloromethyl ether and subsequently trimethylamine or hydroxyethyldimethylamine to prepare the quaternary amine exchanger as is shown below:
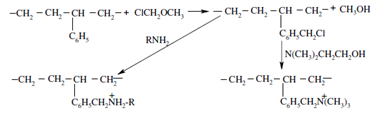
"Weak anion exchanger" "Strong anion exchanger"
Hydrophilic polymers allow the separation of proteins, nucleic acids and other large ionic molecules. A microporosity of those ion exchangers minimizes possible exclusion effects.
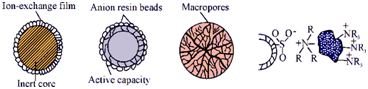
Figure: Various structural types of ion-exchange packings: (a) pellicular with ion-exchange film; (b) exchanger beads coated superficially with porous resin; (c) macroreticular resin bead and (d) anion exchanger surface sulfonated and bonded electrostatically