Separation of ionic and nonionic compounds:
Let us consider the separation of water soluble vitamins, strongly ionic thiamine, non- ionic riboflavin and less ionic pyridoxine and niacinamide by a two step process. In the first step, water/methanol ratio is adjusted to obtain good retention of non-ionic riboflavin and then the organic counterion is added to the eluent to separate three ionic compounds which is affected by the alkyl chain length of the counterion. Thus, thiamine, a quaternary amine shows greatest sensitivity to change in counterion. The optimum separation is achieved with a 50/50 mixture of C-5/C-7 alkyl sulphonic acids as shown in Figure where a mixture of counterions is added to the mobile phase producing retention proportional to the concentration of each counterion.
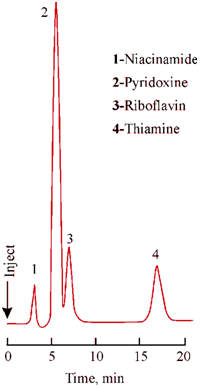
Figure: Separation of ionic and nonionic compounds in a mixture by ion-pair chromatography