Horizontal trends
The chemical trends beside each series are dominated through the increase in nuclear charge and within the number of valence electrons. Previous elements can get the group oxidation state equivalent properly to ions with a noble gas configuration (up to MnVII in the 3d series and RuVIII and OsVIII in 4d and 5d). Rising effective nuclear charge brings an increase in IEs as displayed for the 3d elements in diagram 2. for later elements not only does the group oxidation state become extremely strong oxidizing, but redox potentials for any given states (example M3+/M2+) also increase beside the series, like the extra lattice or solvation energies of the higher state turn into less capable to compensate for the higher IE values.
With increasing IEs comes also a common decline in electropositive character. Early elements in every series are thermodynamically very reactive in the direction of oxygen and other electronegative elements (even though the formation of an inert oxide film may kinetically stop the solid elements from further oxidation). Afterwards elements are less reactive, a trend which culminates in the 'noble' or 'coinage' metals Cu, Ag and Au of group 11. The tendency is exacerbated in the later 4d and 5d elements through the high atomization energies and the elements Ru, Rh, Pd, Os, Ir and Pt create a group known as the platinum metals, frequently taking place together in nature, occasionally as metallic alloys. The change in the electronegativity is as well displayed through dissimilar patterns of chemical stability: where earlier elements of both series usually form more stable compounds with 'harder' anions like fluoride and oxide (and are found in nature in oxide minerals) the afterwards are 'softer' in character and are more often found as sulfides. So the trend along the series gives a link among the chemical characteristics of the pre-transition and post-transition metals.
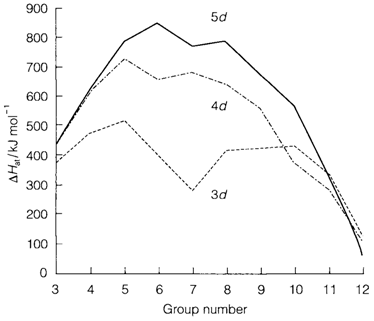
Fig. 1. Standard enthalpies of atomization for elements of the three series.
A common decline in atomic size is other consequence of increasing effective nuclear charge. Figure 2 also depicts the ionic radii of M2+ ions of the 3d series. The supposed decrease across the series is modulated through ligand field effects.