Electron configurations
The neutral atoms' electron configurations are complex and have both d and s electrons within outer shells. For instance, in the 3d series several atoms have the configuration (3d)n(4s)2, whereas n increases from one to 10; chromium and copper are, though, exceptions with (3d)5(4s)1 and (3d)10(4s)1, correspondingly. The configurations rely on a balance of two issues:
(i) 3d orbitals are increasingly stabilized comparative to 4s across the series;
(ii) repulsion among electrons is large in the small 3d orbitals, and so smallest amount of energy in the neutral atom is accomplished despite of (i) by putting one or two electrons in the 4s orbitals.
A significant consequence of this type of balance is that in forming positive ions, 4s electrons are all the time removed first. So for M2+ ions and ones of higher charge, outer-shell electrons are left just in the 3d orbitals. Diagram 2 lists the value of n in the configuration (3d)n for M2+; values for higher charges might be found from these through subtracting the suitable number of electrons (example Ti4+ (3d)0 and Fe3+ (3d)5). These numbers can be employed to interpret the IE trends displayed:
Where I1 and I2 rise quite steadily (with small irregularities resultant from the exceptional configurations of Cr and Cu), the I3 plot depicts a pronounced break after manganese. Along with six or more electrons in the d shell some must pair up, so giving greater electron repulsion and a lower IE than expected from the previous trend (see Topic A5).
Ligand field theory deals with the significant consequences of the progressive filling of the d shell. It is common to identify the d electron number related with the suitable transition metal ion, although the bonding is not assumed to be entirely ionic. For instance, any FeIII compound is assigned the configuration (3d)5, a PtII compound (5d)8 (subsequent to Ni2+ in the same group). In compounds along with very low oxidation states, or with ligands like
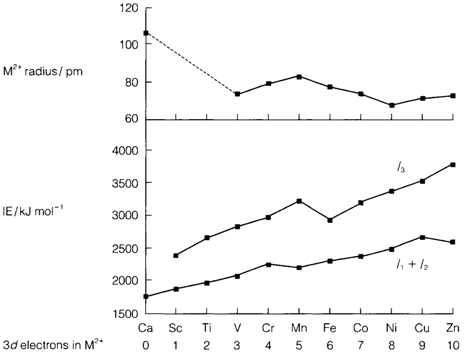
Fig. Data for ions of the elements Ca-Zn showing: radii of M2+ ions, third IE and the sum of first and second IEs, and the (3d)n configurations of M2+.
organic groups in which bonding is largely covalent, a dissimilar electron counting scheme is frequently used. In applying the 18-electron rule one requires in a neutral atom to count the total number of valence electrons, irrespective of whether they are d or s. This is just the group number, so eight for Fe and 10 for Pt. If ligand field arguments are employed for extremely low oxidation states the electrons in the suitable ion are assigned completely to d orbitals.
For instance, a CoI compound would be considered as (3d)8 although the free Co+ ion has the configuration (3d)7(4s) 1. The justification for this method is that the energy balance among d and s orbitals changes on compound formation; what were s orbitals in the free ion become strongly antibonding molecular orbitals in a complex and are no longer taken in the ground state.