Thermodynamic Properties
A property is any (i.e., macroscopic) examinable characteristic of a system. Such distinguishing characteristics of a system are amounts that require to be identified in order to give a macroscopic explanation of the system. A property might be either directly noticeable or indirectly determinable. Most of the quantities are familiar to us from other branches of science, like energy, mass, pressure, density, volume, electric field, magnetic field, and magnetization of matter. The two other properties – temperature and entropy – are exclusive to thermodynamics.
Any grouping of properties, like, the product of pressure and volume, might also be considered as a property. Among the most of possible derived properties, three of them, namely, Enthalpy, Gibbs function & Helmholtz function, are principally helpful.
The definition of a property in thermodynamics has an exclusive meaning. Taking the property pressure, whenever a system has a pressure of p1 at one moment and a value of p2 at the other moment, the change in pressure (p2 – p1) is self-governing of how the change is accomplished. Mathematically talking,
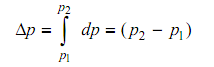
That means that dp is a precise differential, and the integral is not-dependent of the path obeyed. For this purpose, thermodynamic properties are termed as point functions or state functions. A quantity whose value based on the specific path obeyed in going from one state to the other is termed as a path function. The differential of such a quantity is inaccurate.