Scope
The lanthanides and actinides and transition metals have characteristic patterns of chemistry and are treated in Sections H and I. The left over non-transition metals comprise the elements of group 12 even though they are generally part of the d-block, like the d orbitals in these atoms are very tightly bound to be concerned in chemical bonding and the elements do not depict characteristic transition metal properties.
Figure 1 depicts the position of non-transition metals in the periodic table. They come into two classes with considerably dissimilar chemistry. The pre-transition metals include groups 1 and 2 and aluminum in group 13. They are general metals, extremely electropositive in character and approximately invariably found in oxidation states supposed for ions in a noble-gas configuration (example Na+, Mg2+, Al3+). In nature they take place broadly in silicate minerals, even though weathering processes give increase to concentrated deposits of other compounds like halides (example NaCl, CaF2) carbonates (CaCO3) and hydroxides (AlO(OH)).
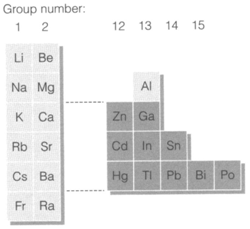
From periods 4-6 in groups the metallic elements following the transition series are post-transition metals. They are than the pre-transition metals less electropositive and are generally found in nature as sulfides rather than silicates. They create compounds with oxidation states subsequent to d10 ions in which s and p electrons have been ionized (example Cd2+, In3+, Sn4+) but these are less ionic in character than subsequent compounds of pre-transition metals. Post-transition metals form stronger complexes than with pre-transition metals in solution. Lower oxidation states (example Tl+, Sn2+) are also general.