Positive ions
The creation of ionic compounds relies on a balance of energies as demonstrated for NaCl. Energy input needed to form ions must be compensated through the lattice energy of the compound. In solution for ions, an identical cycle could be drawn, including the solvation energy than the lattice energy. The second ionization energy comprises an electron from an inner shell and is so large that the extra solvation or lattice energy obtainable with M2+ cannot compensate for it, for group 1 atoms with the (ns)1 configuration. The second ionization energy is additional than compensated through extra lattice energy for group 2 elements with the (ns)2 configuration. So, M2+ compounds are supposed, a solid like CaF(s) consisting a strong tendency to disproportionate.
Diagram 2 gives some data for groups 2 and 12 that are appropriate in understanding the tendencies in pre- and post-transition metal groups. Ionization energies decrease and ion sizes raise, down group 2. Increasing size provides smaller lattice energies, and thus if the electropositive character is to be retained, a decrease in ionization energy is also needed. This occurs in groups 1 and 2, and the electrode potentials depicted in Fig. 2 become slowly more negative for the lower elements.
Group 12 atoms contain the electron configuration ((n-1)d)10 (ns)2 and also form positive ions M2+ by removal of the s electrons. By Filling d shell from Ca to Zn involves an increase of effective nuclear charge that raises the ionization energy and reduces the ionic radius. Lattice energies for Zn2+ are expected to be rather larger than for Ca2+, and the formation of Zn2+ is also assisted through the little lower sublimation energy of metallic zinc. Yet, these issues do not compensate completely for the increased ionization energy, and thus zinc is less electropositive (less negative E? value) than calcium. Ionization energies do not decrease to balance for smaller lattice energies like they do in group 12, and E? values raise down the group on descending group 12. This is especially marked with mercury, in which particularly high ionization energies result from the extra nuclear charge subsequent on filling the 4f shell in the sixth period that is combined with relativistic influences.
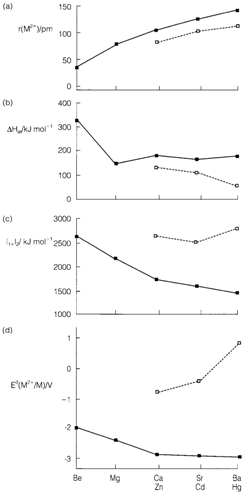
Fig. Data for formation of M2+ ions of groups 2 and 12, showing (a) ionic radii, (b) sublimation enthalpies of the elements, (c) sum of the first two ionization energies, and (d) standard electrode potentials.