Ionic chemistry
Simple monatomic anions are created through only the most electronegative elements, within groups 16 and 17 (example O2-, Cl-). Even though C and N form some compounds that could be formulated in this method (example Al4C3and Li3N), the ionic model is not suitable for these. There are frequently structural variations between oxides or fluorides and the subsequent compounds from later periods. These are partly because of the larger size and polarizability of ions, but rather than oxides the compounds of S, Se and Te are much less ionic.
Several polyanions are known. Those along with multiple bonding are feature of period 2 (example C2-2 and N-3 ); ones with single bonding are frequently more stable for heavier elements (example S2-n), and some create polymerized structures. Simple cations are not a characteristic of nonmetal chemistry but some polycations like O+2 and S2+4 can be created under strongly oxidizing conditions. Complex anions and cations are discussed below.
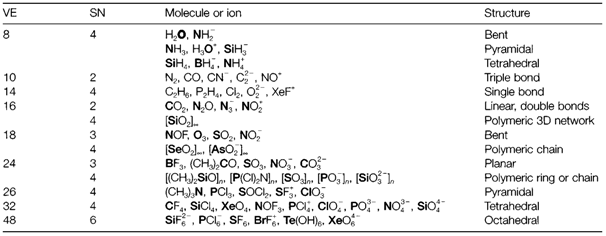
Table 1. A selection of molecules and ions (including polymeric forms) classified as per to the valence electron count (VE) and the steric number (SN) of the central atom shown in bold type